Crystallisation Methodology
Introduction
Solid Solution Formation
Enantiomeric Conglomerates
Kinetic Entrainment and Crystal Twinning
Metastable Zone Width
Polymorphism
Controllable Physical Variables
Dynamic Resolution
References
Introduction
Crystallisation-based methodology is a particular focus of McCague Scientific Consulting. This is for the separation of chiral isomers (enantiomers), the separation of other isomer forms (e.g. geometric isomers of olefins), to the removal of impurities by a crystallisation method, and to access a particular polymorph. In the case of resolution, in the life-sciences it is common for intermediates to be carboxylic acids or amines, which opens the possibility to screen for salt forms having the desired crystalline properties. A database of commercially-available salt-forming agents is used to assist in the selection of an appropriate set of salts to screen for a given substrate. For development of a crystallisation process, a physical-chemistry based approach using mathematical modelling simulation is applied to define the optimal conditions for industrial scale up. This includes understanding ternary phase diagrams. An example of a calculated (simulated) phase diagram for a diastereoisomeric salt (classical resolution) system is shown below. Aside from such screening and resolution development support, the same principles can be used to troubleshoot difficult resolution scenarios, e.g. if a resolution that worked first time breaks down when scaled up, or if an impurity in a pharmaceutical agent can be removed to some extent but not completely by crystallisation. Experience has shown that there is generally rather more complexity to crystallisation-based resolution than is realised by most of its practitioners.
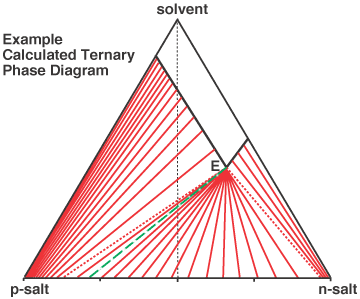
In order to understand a crystallisation-based resolution or purification requires consideration of several
possible scenarios, limitations in the solid-state physical chemistry, or other eventualities, some of the more
important of which are listed below. Advice on these can be provided through consultancy, particularly noting
that the concepts overlap and there is benefit in considering several of the topics simultaneously for a given
crystallisation problem.
If undertaking chiral resolution work (of enantiomers or diastereoisomers)
then a useful calculator is available through the first link below; it relates the enantiomeric (or
diastereoisomeric excesses) of the crystal and liquor phases with the yield after a resolution. If undertaking
purification work, then there is calculator for this also, available by the second link below.
Solid-Solution Formation
When separating compounds of similar molecular structure (e.g. isomers) by crystallisation, a common problem is solid-solution formation whereby the required compound incorporates some of the unwanted one within its crystal structure. The result is that the compound does not initially crystallise out pure, and then the effectiveness of recrystallisation to enhance the purity is limited. The ternary phase diagram shown above accounts for a degree of solid-solution formation; that is why the non-eutectic tie-lines do not lead to diastereoisomerically-pure salt. A dedicated page on theory to quantify the extent of, and consequence of, solid-solution formation in crystallisation resolution is given by this link:
An article on 'a measure of the solid-solution extent useful for crystallisation resolution studies' has been
published as:
McCague, R., Tetrahedron Letters, 2007, 48, 869-872
doi:10.1016/j.tetlet.2006.11.131.
Enantiomeric Conglomerates
A racemic mixture of enantiomers can generally crystallise in one of two ways, either as 'racemic compound' in which the crystals contain a lattice with a regular arrangement of both enantiomers in equal amounts, or as a 'conglomerate' in which there is a physical mixture of crystals, each crystal comprised of one or other single enantiomer [Ref 1.]. This difference is represented by the illustration below, and the possibilities can be characterised and distinguished by their binary phase diagram of composition versus melting point or solubility. Formation of a 'racemic compound' is the more common, but when a conglomerate is found it has the advantage of enabling an efficient elevation of the enantiomeric purity, since generally the whole of any enantiomeric excess should concentrate into the crystals, leaving racemic liquors. Furthermore, there is sometimes the possibility of developing a direct resolution of a conglomerate racemate by sequential kinetic entrainment of the single enantiomers. In a chiral synthesis, it is certainly worthwhile to check any enantiomeric/racemic compounds on the pathway to see if any are conglomerates and therefore lend themselves to ready stereopurification. Additionally where a resolution needs to be undertaken, if the compound of interest is capable of forming salts (e.g. an amine or carboxylic acid) then it is often worthwhile to screen appropriate salt-forming agents (achiral) until a suitable conglomerate is found. In particular, given the frequent problem of solid-solution formation during diastereoisomer salt (classical) resolutions, a means to obtain high enantiopurity material is to make no attempt to recrystallize the initially formed diastereo-enriched salt from a classical resolution, rather to exchange it to a salt with an achiral agent that is a conglomerate and recrystallise that.
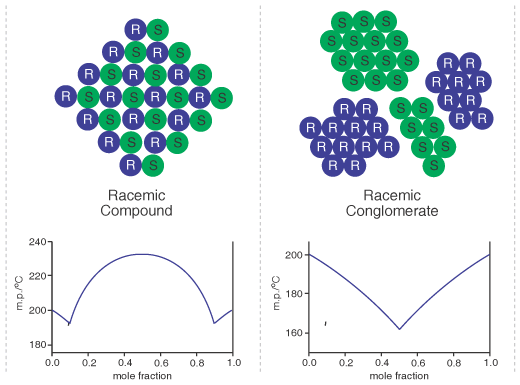
Aside from the existence of racemic compounds versus conglomerates, there can in addition be solid-solution formation, and simulated phase diagrams can be calculated to take this into account - see Solid Solution Theory. In the extreme case, the enantiomers can distribute at random within a given crystal lattice to give what is termed a pseudoracemate. This is likely to occur if the enantiomers are of very similar spatial structure, an example is racemic camphor.
Kinetic Entrainment and Crystal Twinning
Above is mentioned the possibility of direct resolution of a conglomerate racemate by means of kinetic
entrainment. In this technique, an appropriately supersaturated solution of the racemate is made and
crystals of one enantiomer added. Then for favourable conglomerates, that enantiomer crystallises further
whilst the other remains in solution. So the liquors become enriched in the other isomer. Now further
racemate can be added and the mixture entrained with the other isomer. By repeating this process of
harvesting crystals of alternate enantiomers and charging the solution with more racemate in-between, a
racemic mixture can in principle be entirely separated into its two enantiomers. This can be a highly
economic manufacturing method but needs careful control of concentrations, stirring rate, temperatures, and
timing to be effective. Typical yields from a successful conglomerate entrainment might be in the range of
4%-30% of single enantiomer harvested per racemate added per cycle, and given that some can be operated in a
highly concentrated solution (or even in the melt) favours the process economics. For reasons of common
ion effect, the best entrainments are generally found with salts rather than with neutral conglomerates, so
another reason to screen for, and work with salts where possible. McCague Scientific Consulting is
available to help with development of a such an entrainment resolution of a racemic conglomerate.
Sometimes there is celebration at discovering a conglomerate, yet attempted entrainment-resolution fails because
of crystal twinning. That is when the crystal surface of one enantiomer can act as a template to induce
crystallisation of the other. If the extent of twinning is high then it is not possible to achieve any
significant supersaturation of one isomer whilst the other is crystallising. The twinning might be
direct; between the crystals of wanted and unwanted isomer, or it might be indirect. With indirect
twinning the crystallising material initiates nucleation of a further crystal form that in a relay effect causes
nucleation of the unwanted isomer. The further crystal form could be a metastable racemic-crystal of
the compound being entrained [Ref 2.]. When screening salt forms, it is aimed to
identify several conglomerates, then at least one should hopefully not suffer this twinning problem.
The technique of direct resolution by entrainment of conglomerate racemates is well known in the chiral
process technology community. What is less well known, and often overlooked, is that similar principles
can be applied to diastereoisomeric salts, i.e. to entrain classical resolutions with the desired isomer so that
the preferred isomer can be crystallized selectively to a higher extent than the equilibrium solubilities would
suggest. In favourable cases the level of entrainment can be considerable; an example is the resolution of
the anti-inflammatory agent etodolac with N-methylglucamine (meglumine)
[Ref 3.] . Examination of the method in the patent suggests the solubility of the
diastereoisomers is similar, however seeding of a supersaturated solution with (R)-diastereoisomer gave 32%
yield of this salt, and subsequent seeding of the liquors with the (S)-diastereoisomer gave a 32% yield also of
this other salt. By employing diastereoisomer entrainment may allow a higher yield of the required isomer with a
given resolving agent; alternatively it may enable use of a more readily available/cheaper resolving agent and
make a resolution more economically feasible. In principle it could allow a diastereoisomer mixture to be
separated completely by continuous sequential entrainments.
Just as there is an issue of crystal
twinning in the case of racemic conglomerates, the same is the case of diastereoisomer salts if entrainment is
contemplated. In any case in the development of a classical resolution it is worth to identify if one
diastereoisomer induces the crystallisation of the other or not.
Various entrainment scenarios can be
modelled mathematically. This is discussed in detail by this link:
Metastable Zone-Width
A solution of organic compound can usually sustain some degree of supersaturation for some time without crystallisation taking place. The extent to which this happens is known as the metastable zone and is conveniently measured as a temperature difference, i.e. by how much a saturated solution can be cooled before crystallisation starts. Given the temperature-solubility profile, it may however be more meaningfully measured as the excess concentration of material over the saturated solution, or as a percentage supersaturation (concentration relative to that in the saturated solution). Determination of metastable zone width is critical in the development of a resolution by entrainment of a conglomerate [Ref. 4], because if the solution is too supersaturated the unwanted isomer will crystallise spontaneously, and in any case the rate of crystallisation will lack the necessary control. Consideration of metastable zone-width can however be an important factor in other types of crystallisation processes; by keeping within the zone, nucleation is avoided but crystal growth is encouraged so that larger, more easily handled crystals should be obtained. Also in classical resolution, by ensuring the metastable zone of the unwanted diastereoisomer is not exceeded, can maximise the crop of the desired isomer. As indicated above, potentially the metastable zone of a diastereoisomer salt can be large so it is worth finding out if this is the case. An interesting recent paper concerning racemic versus single enantiomer ketoprofen [Ref. 5.] shows that the metastable zone width for its racemate is much wider than that of the single isomer, and explains why the single enantiomer can be difficult to purify.
Polymorphism
Polymorphism is a hot topic in pharmaceutical formulation; different crystal forms of the same drug entity can have different solubility, oral-bioavailability, stability, and mechanical properties. Polymorphism is also an important issue in resolution since either the desired component, or the unwanted component (impurity) could exist in more than one crystalline form. In particular, diastereoisomer salts can be reluctant to crystallise, and if they do crystallize the thermodynamically most favoured form does not necessarily appear straight away. In particular, the 'classical' method of resolution screening whereby samples of a concentrated racemate solution are taken with various resolving agents does not give the best chance for the most stable crystal form of the diastereoisomer to deposit, or indeed for anything to crystallise at all. It is advocated that it is better to make some pure diastereoisomer first and study its crystallinity properties before proceeding to whether it will resolve from the other isomer. That said, the possibilities of crystal twinning (see above) can complicate the picture of polymorphism in crystallisation resolution studies. Either wanted or unwanted diastereoisomer crystals might twin with and therefore promote a morphology of the other isomer that is not seen if that other isomer crystallises alone. If both diastereoisomers are polymorphic then the system might deliver results in which the form of the first which crystallizes will dictate the form of the other diastereoisomer (in theory). So, polymorphism is clearly a factor that must be considered when developing any crystallisation-based resolution or purification procedure.
Controllable Physical Variables
In developing a crystallisation resolution process, there are several variables that can be imposed on the compound mixture externally. One is temperature; a resolution may perform differently if operated at a different temperature range. Indeed if a compound is polymorphic, a different temperature may determine which crystal form is thermodynamically favoured. Other factors affecting a resolution are the solvent chosen and concentration of operation. If there are polymorphs a particular solvent may favour the deposition of one over another. A particular solvent may also give rise to solvate formation, and possibly at one temperature of operation or not another. Solvate formation might affect crystallisation of either the desired compound or of that to be removed (the impurity). Moreover, these additional factors can become critical in the case of crystallisation processes relying on kinetic phenomena, such as in carrying out entrainment of a desired crystal form, and in order to obtain crystals of a suitable mechanical form for handling.
Crystallisation Induced Asymmetric Transformation (Dynamic Resolution)
Lastly it should be mentioned that a disadvantage of racemate resolution in process chemistry is if the undesired enantiomer is discarded then the best yield obtainable is only 50%. Sometimes the off-isomer can be collected also, racemized back to racemate and then used again in a subsequent resolution. However, this can cause process and regulatory issues if the recycled material is not of the same purity profile as the fresh synthetic racemate. The ideal process would have the racemisation of off-isomer taking place during the resolution, and then crystallisation of a preferred form can drive the equilibrium over to the desired material. Many examples of such dynamic resolutions now exist in the literature [Ref 6.]. In terms of development of such a process though, there is the extra challenge of finding compatible conditions for both the racemisation and crystallisation together, and it is especially demanding if the required crystallisation depends on kinetic control (e.g. by an entrainment) rather than by deposition of a thermodynamically favoured crystal form.
References
- J. Jacques, A. Collet, S. H. Wilen, Enantiomers, Racemates and Resolutions, Krieger, Malabar, 1981, reprinted 1994.
- M.-N. Petit and G. Coquerel, Mendeleev Communications, 2003, 13, 95-96.
- B.M. Adger. U.C. Dyer, M. Woods, J.F. Andrews and H.F. Baker, European Patent Appl. EP 0755398 B1/2002
- A.A. Rodrigo, H. Lorenz and A. Seidel-Morgenstein, Chirality, 2004, 16, 499-508.
- Y.H. Lu and C.B. Ching, Chirality, 2006, 18, 239-244.
- See the work by Merck on a process for the synthesis of their anti-emetic drug aprepitant: M.M. Zhao, J.M. McNamara, G.H. Ho and 10 others, J. Org. Chem., 2002, 67. 6743-7; K.M.J. Brands, J.F. Payack, J.D. Rosen and 19 others, J. Am. Chem. Soc., 2003,125, 2129-35.